pf3 electron pair geometry|electron pair geometry chart : Tagatay The Lewis structure is drawn using eight dots of valence electrons around the symbols of the atom with lines showing bond formation. PF3 is a tetra-atomic molecule where phosphorus donates three valence electrons, and three fluorine atoms . Tingnan ang higit pa Meilleur processeur socket 1155 LGA Intel Core i7-3770K IvyBridge. Le microprocesseur Intel core i7-3770 Ivybridge a déjà trouvé sa place dans les ordinateurs de nombreux utilisateurs. Un tel attrait est bien justifié par les performances qu’offre ce dispositif. Ce processeur dote votre ordinateur d’un niveau de performances hors normes.
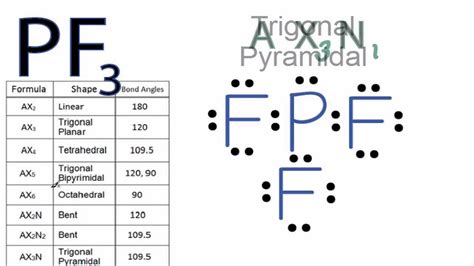
pf3 electron pair geometry,The geometrical structure of the tetra-atomic Phosphorus Trifluoride (PF3) molecule is studied with the help of the Valence Shell Electron Pair Repulsion (VSEPR) theory. This theory explains that the bond angle between the fluorine-phosphorus-fluorine (F-P-F) is 97°. This angle makes . Tingnan ang higit paAs per this rule, the maximum number of valence electrons an atom can have is eight. One phosphorus atom has five valence . Tingnan ang higit pa
The electrons present in the outermost shell of an atom are called valence electrons. Because they are present in the outermost . Tingnan ang higit papf3 electron pair geometryHybridization is a method of combining atomic orbitals of the same atom to produce new orbitals which are called hybrid orbitals. To figure out the hybridization . Tingnan ang higit pa
The Lewis structure is drawn using eight dots of valence electrons around the symbols of the atom with lines showing bond formation. PF3 is a tetra-atomic molecule where phosphorus donates three valence electrons, and three fluorine atoms . Tingnan ang higit pa
pf3 electron pair geometry electron pair geometry chart A quick explanation of the electron geometry of PF3. The electron geometry for PF3 it Tetrahedral. It is important to note that you must first draw the .

A quick explanation of the molecular geometry of PF3 including a description of the PF3 bond angles. Looking at the PF3 Lewis structure we can see that .
The electron geometry for PF3 is tetrahedral as it central has 4 regions of electron density. Lewis dot structure of PF3 contains 1 lone pair on the central .
Lewis structure of PF3 contains three single bonds between the Phosphorus (P) atom and each Fluorine (F) atom. The Phosphorus atom (P) is at the center and it is .
We show you how to draw the Lewis structure and determine the moleculargeometry for phosphorus trifluoride (PF3).
In calculating electronic geometry we use the Valence Shell Electron Pair Repulsion (VSEPR) model, which states that the lowest geometry for electronic orbitals around a positive nucleus is for the orbitals to be .
Total electron pairs = total valence electrons ÷ 2. So the total electron pairs = 26 ÷ 2 = 13. Third, determine the central atom; We have to place the least .When a central atom has two lone electron pairs and four bonding regions, we have an octahedral electron-pair geometry. The two lone pairs are on opposite sides of the octahedron (180° apart), giving a .
What is the molecular geometry of the P F 3 molecule? Chemistry Molecular Orbital Theory Molecular Geometry. 1 Answer. Dwight. Dec 15, 2016. Trigonal .
electron pair geometry chart The Lewis structure of PF3 shows that the phosphorus atom is bonded to three fluorine atoms. Each bond in PF3 is a polar covalent bond due to the difference in electronegativity between phosphorus and fluorine. However, the molecular geometry of PF3 is trigonal pyramidal, which results in an overall nonpolar molecule. An explanation of the electron geometry for the BF3 (Boron trifluoride) . The electron geometry for the Boron trifluoride is also provided.The ideal bond ang.Question: PF3 Bonding Atoms: Non-Bonding Electron pairs (central atom): Electron Pair Geometry: Select an answer Molecular Shape: Select an answer Polar: yes :F: :F—P P—F: Show transcribed image text. There are 3 steps to .
So we have to only mark the remaining ten electron pairs as lone pairs on the sketch. Also remember that phosphorus is a period 3 element, so it can keep more than 8 electrons in its last shell. And fluorine is a period 2 element, so it can not keep more than 8 electrons in its last shell. Always start to mark the lone pairs from outside atoms.Thus, the electron-pair geometry is tetrahedral and the molecular structure is bent with an angle slightly less than 109.5°. In fact, the bond angle is 104.5°. Figure \(\PageIndex{9}\): (a) H 2 O has four regions of electron density around the central atom, so it has a tetrahedral electron-pair geometry. (b) Two of the electron regions are .
Phosphorus Trichloride: Phosphorus trifluoride is the name of PF 3.It's a gas that is known for its toxicity. We will use valence shell electron pair repulsion (VSEPR) theory to determine its molecular geometry.

The electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{CH_4}\). In the ammonia molecule, one of the electron pairs is a lone pair rather than a bonding pair. Although the lone pair is not visible, it will affects the location and bond angles among other atoms in the molecule. Example \(\PageIndex{1}\) Determine the Electron Group Arrangement and Molecular Geometry about the central atom(s) in a) OF 2 and b) CH 3 CN.. Solution. a) The Lewis dot structure of OF 2 is (leaving off the lone pairs on the non-central F atoms.). There are 2 atoms and 2 lone pairs attached to the central O atom, for a total of 4 "things .
pf3 electron pair geometry|electron pair geometry chart
PH0 · pf3 lewis structure molecular geometry
PH1 · how to determine electron geometry
PH2 · electron pair geometry chart
PH3 · electron geometry vs molecular geometry
PH4 · Iba pa